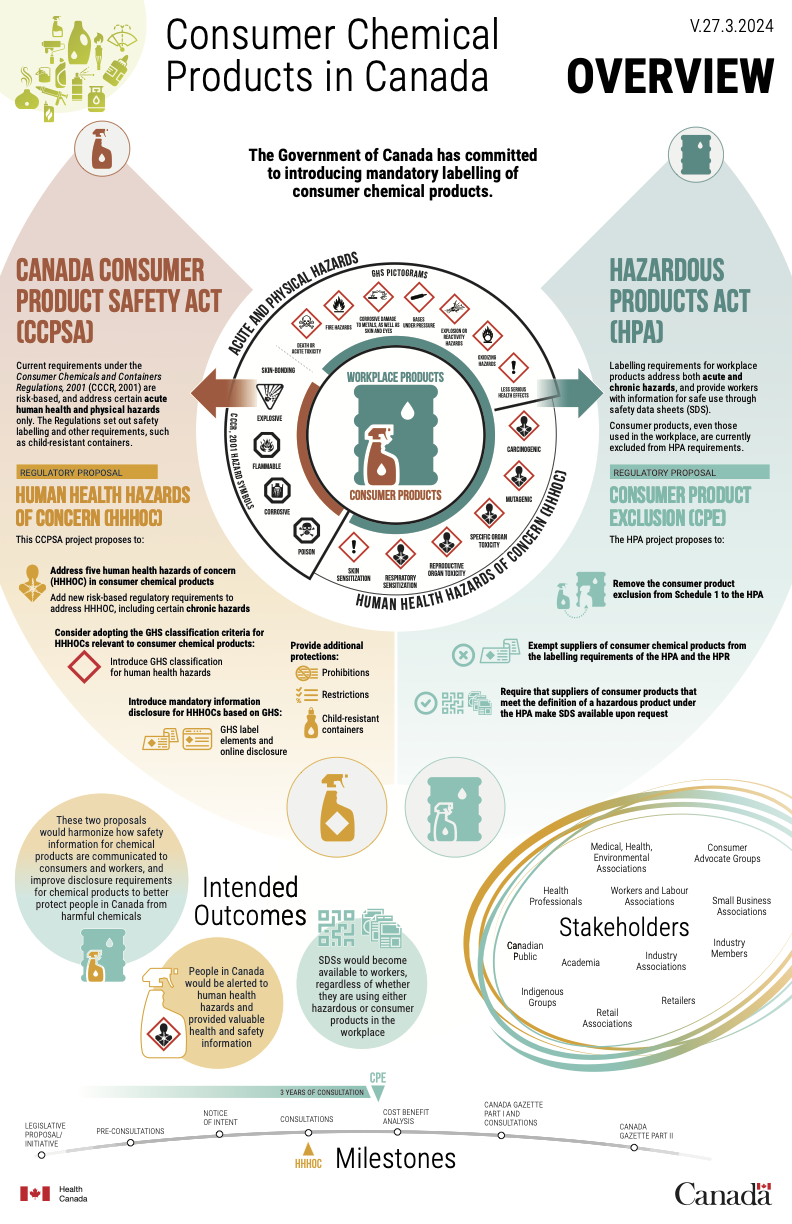
Health Canada held a multi-stakeholder workshop on the Workplace Hazardous Products Program on November 7, 2024. During this workshop, significant attention was paid to the Consumer Chemicals and Containers Regulations (CCCR). This important regulation under the Canada Consumer Product Safety Act (CCPSA) dictates the requirements that chemical products and their containers must meet when they are made available to consumers, to prevent accidents resulting in injuries or deaths. At this workshop, Health Canada presented its draft plan for amendments to the 2001 regulations. Amendments to the Hazardous Products Act (HPA) and the Hazardous Products Regulations (HPR) affecting consumer products were also presented.
Inclusion of Chronic Health Hazards in the CCCR
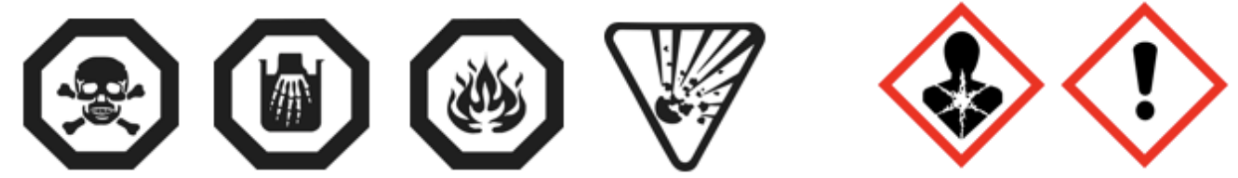
This is probably the most significant of all the proposed changes to the CCCR. In its 2001 form, the regulations consider physical and acute health hazards to consumers that fall into the following categories:
-
Corrosive
-
Toxic
-
Flammable
-
Quick skin-bonding adhesives
-
Pressurized containers
![Proposed Changes to CCCR: Moving Towards GHS for Consumer Products]()
Source: Workplace Hazardous Product Program multi-stakeholder workshop, Health Canada, 2024-11-07.
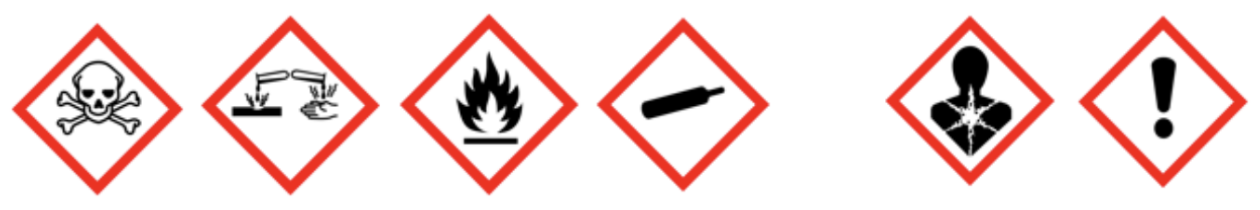
However, the regulations do not consider the risks of adverse health effects associated with medium- or long-term exposure. It should be noted that, when they were adopted in 2001, there were no harmonized classification criteria for these hazards, as currently prescribed by the UN Globally Harmonized System (GHS). With the current state of knowledge, it appears that the CCCR could go much further to protect Canadian consumers by including classification criteria for the following human health hazards of concern:
-
Carcinogenicity (induction of cancer)
-
Germ cell mutagenicity (heritable gene mutations)
-
Reproductive toxicity (adverse effects on sexual function, fertility or developmental toxicity in offspring)
-
Specific target organ toxicity (adverse effects on target organs after single or repeated exposure)
-
Respiratory or skin sensitization (allergic reaction or hypersensitivity)
Health Canada published a notice of intent in July 2023 to seek stakeholder input on a potential regulatory change. The notice of intent proposed two approaches to include human health hazards of concern in the regulatory framework:
- The addition of the chronic hazard pictograms of the GHS to the already existing pictograms of CCCR, to get a hybrid system of pictograms
![CCCR pictograms]()
- The replacement of the current CCCR pictograms by those of the GHS
![GHS pictograms]()
During the consultation period, most respondents (71%) indicated that the GHS was effective in addressing the risks associated with adverse health effects. Furthermore, nearly three times as many respondents indicated that they preferred replacing the current pictograms over a hybrid approach (41% versus 14%). The remaining respondents proposed alternative approaches, such as the status quo and harmonization with the United States. Both alternatives were considered but were not retained by Health Canada.
Guided by these answers, Health Canada has continued its activities by conducting public opinion research and testing the public's knowledge of the current pictograms of the CCCR and the equivalent pictograms of the GHS. The recognition and interpretation of the proposed new health hazard and exclamation mark pictograms were also studied.
These studies revealed there is not a significant difference between the public’s understanding of the pictograms in the CCCR and those in the GHS with respect to acute hazards of toxicity, corrosivity and flammability. However, understanding of the health hazard and the exclamation point pictograms was low among respondents, with 41% and 18% of understanding respectively. This information clearly highlights that, in the event these pictograms are added to the CCCR, a plan will need to be put in place to inform the public about the meaning of these pictograms and the hazards associated with them.
With all this information in mind, Health Canada now plans to move forward with a reform of the CCCR that will replace the current pictograms with those of the GHS. The current plan includes several stages of consultations and information sessions on the project until June 2026.
| Topic Areas for the Proposal |
Tentative Timelines |
1. Overview of:
- stakeholder feedback received on the NOI under the CCPSA
- public opinion research summary, and
- analysis for the selected regulatory approach i.e., a full replacement of the CCCR, 2001 with a risk-based framework based on the GHS
|
Information session with opportunity for questions and answers - January to March 2025. |
| 2. Consumer chemical product scope, exclusions, definitions, record-keeping requirements, and other general provisions |
Consultations held June to September 2025 |
| 3. Hazard classification and risk characterization of consumer chemical products |
Consultations held October to December 2025 |
| 4. Information disclosure requirements including labelling |
Consultation held January to March 2026 |
| 5. Additional protections (e.g., prohibitions, restrictions, child-resistant containers, etc.) |
Consultations held April to June 2026 |
Source: Workplace Hazardous Product Program multi-stakeholder workshop, Health Canada, 2024-11-07.
Amendment to the Hazardous Products Act
Currently, consumer products as defined in the CCPSA are listed in Schedule 1 of the Hazardous Products Act (HPA). For this reason, the prohibition in the HPA against having a hazardous product in a workplace that is not identified in accordance with WHMIS does not apply to consumer products.
Health Canada indicates that more and more consumer products are used in workplaces, while no indication of chronic risks related to human health is indicated on these products. This poses a risk to workers who could be regularly exposed to them without knowing the potential adverse health effects.
To better protect these workers, Health Canada is proposing to make the following amendments:
- Remove consumer products from Schedule 1 of the HPA
- Exempt, in the Hazardous Products Regulations (HPR), suppliers of consumer products from WHMIS labelling requirements
- Require suppliers of consumer products that meet the definition of a hazardous product under the HPA to provide an SDS upon request.
These amendments would not only require suppliers to produce or make available SDSs, but would also require employers to make available in the workplace the SDSs of consumer products used or stored. Indeed, article 16 of the Règlement concernant l’information sur les produits dangereux indicates that, if a data sheet must be produced under the HPA and the HPR, it must be available in the workplace (RIPD, sect. III, art. 16).
Conclusion
Although Health Canada has clearly indicated its desire to move forward with its proposed CCCR reform, it will take several more years before reaching the point of regulatory change. Indeed, as mentioned above, Health Canada is planning consultation sessions until July 2026 before drafting a regulatory amendment that can be filed. In the ongoing goal of better protecting the health of Canadians using hazardous products, whether in their workplaces or homes, the federal agency seems firm in its intention to revise the regulations.
Do you want to sell a consumer product and have questions about the process? Do not hesitate to call the experts at Kalium Solutions!
Source: Health Canada